
The US FDA New Drug Approvals in September 2024
Shots:
-
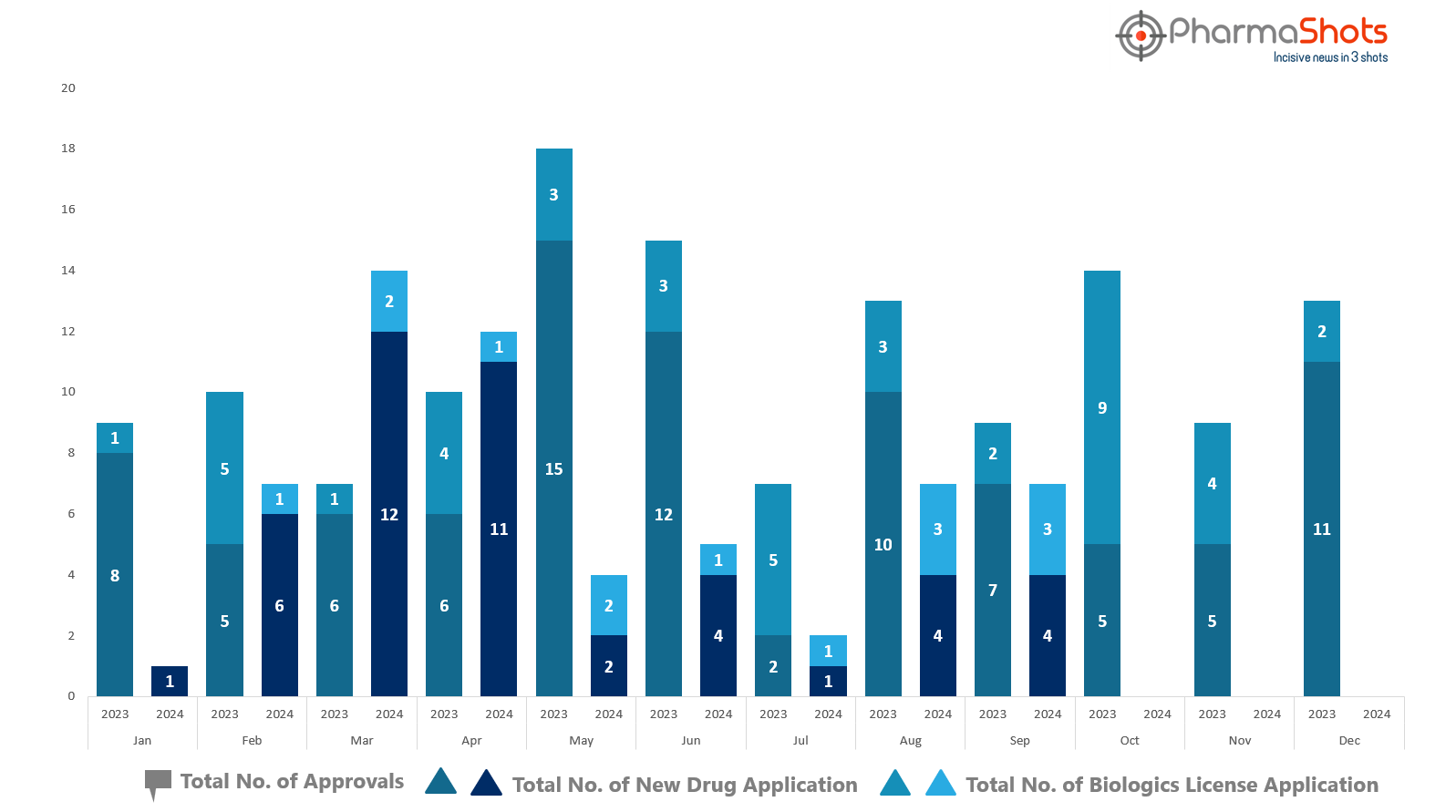
PharmaShots has compiled a list of US FDA-approved drugs in the month of September 2024
-
The US FDA has approved a total of 7 new drugs including 4 new molecular entities and 3 biologics leading to the treatment of patients and advances in the healthcare industry
-
The major highlighted drugs were Roche’s Tecentriq Hybreza & Ocrevus Zunovo for the treatment of various cancers as well as relapse & progressive multiple sclerosis, respectively
1. Roche’s Tecentriq Hybreza Receives the US FDA’s Approval for Cancer Treatment
Product Name: Tecentriq Hybreza
Active ingredients: Atezolizumab and Hyaluronidase-tqjs
Company: Roche
Date: Sep 12, 2024
Disease: Lung, Liver, Skin & Soft Tissue Cancers
Shots:
- The US FDA has approved Tecentriq Hybreza (SC) to treat all IV indications, incl. lung, liver, skin & soft tissue cancers. Regulatory reviews across other regions are underway
- The approval was supported by P-IB/III (IMscin001) study assessing Tecentriq Hybreza (SC) vs Tecentriq (IV) in locally advanced or metastatic NSCLC patients (n=371) failed on Pt therapy and P-II (IMscin002) assessing patient preference b/w SC vs IV in those (n=179) with PD-L1+ve resected Stage II-IIIB NSCLC & untreated Stage IV NSCLC
- IMscin001 trial showed consistent Tecentriq levels in blood with safety profile aligning with IV formulation. In the IMscin002 trial, 71% preferred SC over IV, with 79% opting to continue treatment with it
Product Name: Ebglyss
Active ingredient: Lebrikizumab-lbkz
Company: Eli Lilly
Date: Sep 13, 2024
Disease: Moderate-to-Severe Atopic Dermatitis
Shots:
- The US FDA has approved Ebglyss to treat moderate-to-severe AD in adults & children (≥12yrs.) weighing 88 pounds (40kg). Lilly holds its exclusive rights outside the EU, while Almirall has licensed rights in the EU
- The approval was supported by P-III (ADvocate 1 & 2) studies of Ebglyss alone and P-III (ADhere) study of Ebglyss + topical corticosteroids in over 1000 adults & children (12-18yrs.) with mod. to sev. AD
- ADvocate 1 & 2 studies showed clear or almost-clear skin in 38% vs 12% (77% maintained it for 1yr. with QM dosing & 48% maintained it after switching to PBO) and 43% vs 12% had itch relief at 16wks. (85% maintained for 1yr. with QM dosing & 66% maintained it post switching to PBO)
Product Name: Ocrevus Zunovo
Active ingredients: Ocrelizumab & Hyaluronidase-ocsq
Company: Roche
Date: Sep 13, 2024
Disease: Relapse & Progressive Multiple Sclerosis
Shots:
- The US FDA has approved Ocrevus Zunovo (ocrelizumab & hyaluronidase-ocsq), a twice-a-year, 10-minute SC injection, to treat relapsing multiple sclerosis (RMS) and primary progressive multiple sclerosis (PPMS)
- The approval was supported by the pivotal P-III (OCARINA) study assessing the PK, safety, clinical & radiological efficacy of the Ocrevus SC vs IV in patients (n=236) with RMS or PPMS
- The study depicted that SC Ocrevus Zunovo had similar blood levels, safety & efficacy to the IV form in RMS & PPMS. It suppressed relapse activity & MRI lesions by 97% over 48wks. Over 92% of them preferred SC formulation
Product Name: Miplyffa
Active ingredient: Arimoclomol Citrate
Company: Zevra Therapeutics
Date: Sep 20, 2024
Disease: Niemann-Pick Disease Type C
Shots:
- The US FDA has awarded priority review voucher & approved Miplyffa (47mg to 124mg, oral, TID) + miglustat to treat neurological manifestations in adults & pediatrics (≥2yrs.) with NPC, with its launch planned within 8-12wks.
- Approval was based on trial of Miplyffa + miglustat vs PBO in NPC patients (2-19yrs.), showing halted disease progression with 0.2 vs 1.9-point reduction at 12mos. Data from a 48mos. OLE trial provided additional evidence of improved outcomes
- Zevra has launched AmplifyAssist, offering insurance education, copay assistance, disease information, therapy management counseling & support for prescription refills for the eligible patients in need
5. IntraBio’s Aqneursa Receives the US FDA’s Approval for Treating Niemann-Pick Disease Type C
Product Name: Aqneursa
Active ingredient: Levacetylleucine
Company: IntraBio
Date: Sep 24, 2024
Disease: Niemann-Pick Disease Type C
Shots:
-
The US FDA has granted approval to Aqneursa (levacetylleucine) for treating adults & pediatric patients (≥15kg) with neurological manifestations of Niemann-Pick disease type C (NPC)
-
Approval was supported by pivotal P-III (IB1001-301) study assessing the effect of Aqneursa vs PBO on neurological symptoms & functioning in pediatric (≥4yrs.) & adults (n=60) with confirmed NPC
-
Study reached all 1 & 2EPs, depicting improved neurological symptoms & functional benefits at 12wks. as well as greater improvement in fSARA scores, with a mean difference of -0.4. Results were published in the New England Journal of Medicine
Product Name: Cobenfy
Active ingredients: Xanomeline Tartrate and Trospium Chloride
Company: BMS
Date: Sep 26, 2024
Disease: Schizophrenia
Shots:
- The US FDA has granted approval to BMS’ Cobenfy for treating schizophrenia in adults, based on its EMERGENT studies
- The EMERGENT studies comprised of P-III (EMERGENT-2 & 3) trials, evaluating the safety, efficacy & tolerability of Cobenfy vs PBO in schizophrenic adults for over 5wks., as well as two open-label trials assessing long-term safety & tolerability of Cobenfy for over 1yr.
- Studies reached the 1EPs, showing reduced schizophrenia symptoms with 9.6 (-21.2 vs -11.6 in EMERGENT-2) and 8.4 (-20.6 vs -12.2 in EMERGENT-3) point reductions in PANSS total scores at wk.5. EMERGENT-2 study also depicted a change of 0.6 (-1.2 vs -0.7) in CGI-S score at wk.5 (2EP)
Product Name: Flyrcado
Active ingredient: Flurpiridaz F-18
Company: GE HealthCare
Date: Sep 27, 2024
Disease: Coronary Artery Disease
Shots:
- The US FDA has approved Flyrcado injection as a PET MPI agent to diagnose CAD, with its launch planned in early 2025
- The effectiveness of Flyrcado was assessed under the P-III (AURORA) study in comparison with both invasive coronary angiography as a standard and SPECT MPI to identify CAD
- In addition, GE HealthCare secured exclusive global commercialization rights of the flurpiridaz F 18 from Lantheus in 2017, funding its development until approval. Lantheus will partner on commercialization through a joint steering committee & is entitled to get royalties based on sales milestones
Related Post: Insights+: The US FDA New Drug Approvals in August 2024
Tags

A passionate content writer with expertise in delivering high-quality and engaging content, Dipanshu is a keen reader and a versatile writer. Dipanshu dedicatedly covers news ranging from biopharma, life sciences, biotech, and MedTech to diagnostics and animal health companies, FDA, EMA, and biosimilar approvals. He can be contacted at connect@pharmashots.com














